Up to 256 cash back Draw the Lewis structures including resonance structures for the acetate ion CH 3 COO-For each resonance structure assign formal charges to the atoms that have formal charges. Include all valence lone pairs in your answer.

5 The Two Lewis Resonance Structures Of The Acetate Anion Viz The Download Scientific Diagram
Yeah we can draw the resonant structure by shifting the electrons that are extra on our negative oxygen atom to form a double.
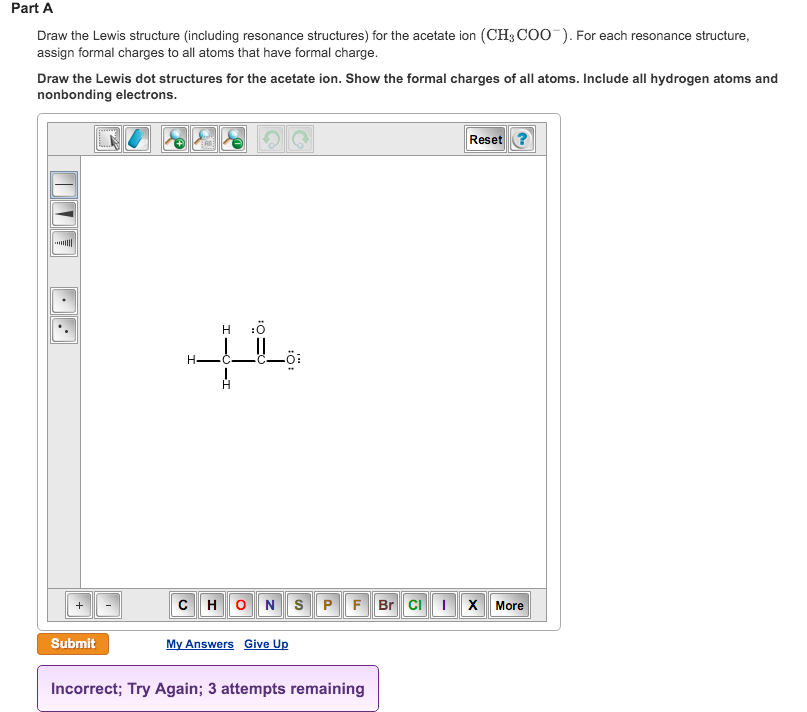
. Assign non-zero formal charges to each atom for each resonance structure. Draw the Lewis structure including resonance structures for the acetate ion CH3COO-. I quickly take you through how to draw the Lewis Structure of CH3COO- Acetic ion.
For each resonance structure assign formal charges to all atoms that have formal charge. Oxygen atom which has made a double bond with carbon atom has two lone pairs. Draw the lewis structure including resonance structures for the acetate ion ch3coo.
A NO3 nitrate b CH3COO acetate c N3 azide. Draw the Lewis dot structures for the acetate ion. For each resonance structure use square brackets to denote the overall charge.
Well put the Carbons next to each other. For each resonance structure assign formal charges to. Draw one structure per sketcher.
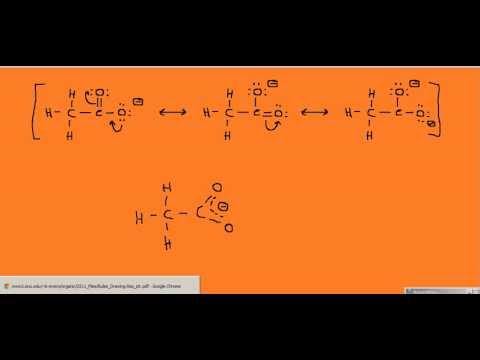
This was an in structure has formal charges -1 on the oxygen with the negative charge zero on the oxygen thats double bonded and zero on both of the carbons. For each resonance structure assign formal charges to all atoms that have formal charge. Explicitly draw all H atoms.
RESONANCE STRUCTURE OF CH3COO- - 1177172 Answer. Do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule. Get the answer to your homework problem.
Do not include overall ion charges or formal charges in your drawing. Include curved arrows that indicate the movement of electrons between each resonance structure. The resonance structure of acetate ion is given below.
Draw the Lewis structure including resonance structures for the acetate ion CH3COO. Resonance occurs when the electrons are. Explicitly draw all H atoms.
The resonance structure of acetate ion is given below. Draw all resonance structures for the acetate ion ch3coo Coffin nails have become the most popular manicure trend right this moment which can be going nowhere promptlyBlack appears to be perfect with any color. Other oxygen atom has a -1 negative charge and three lone pairs.
Cid 6421 trichloroacetic acid date s. For each resonance structure assign formal charges to all atoms that have formal charge. If you recognize this the Lewis structure is much easier to draw.
Do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule. Draw All Resonance Structures For The Acetate Ion Ch3coo. There is a double bond between carbon atom and one oxygen atom.
1 a Draw the two Resonance Structures that describe the bonding in the acetate ion CH3COO-. Draw the Lewis structure including resonance structures for the acetate ion CH3COO. This free chemistry help video tutorial shows you how to create and understand resonance structures for the acetate ion.
For the CH3COO- Lewis structure we have a total of 24 valence electrons. We will draw the LewiS structure for all of the resonant structures and include the formal charges. For each ion below draw all reasonable resonance structures linked by resonance arrows.
For each resonance structure assign formal charges to all atoms that have formal charge. Draw one structure per sketcher. Nonetheless it doesnt enable it to be any less attractive.
Two stable resonance structures also can be drawn for acetate ion. Resonance structure is defined as the structure in which two forms of a molecule have same chemical connectivity but the distribution of electrons around the structure are different. Drawing the Lewis Structures for CH 3 COO-See the Big List of Lewis Structures.
Determine the central atom in this molecule. For the CH3COO- Lewis structure we have a total of 24 valence electrons. Well put an Oxygen on the end here and well put another Oxygen here.
Ionic and Covalent Bonds. Draw the Lewis dot structures for the acetate ion. Draw all resonance structures for the acetate ion CH3COO.
B What is the hybridization of the C in the COO- group. Draw all resonance structures for the acetate ion CH3COO-. Draw the Lewis structure including resonance structures for the acetate ion CH3COO-.
Do not include overall ion charges or formal charges in your drawing. Include all valence lone pairs in your answer. Draw the Lewis structure including resonance structures for the acetate ion CH3COO-.
For each resonance structure assign formal charges to. 18-6 12e-6 lone pairs. C Select one of the two Resonance structures and write out its Bonding Scheme.
Draw the Lewis structure including resonance structures for the acetate ion CH3COO. I also go over hybridization shape sigma pi bonding and bond angles.
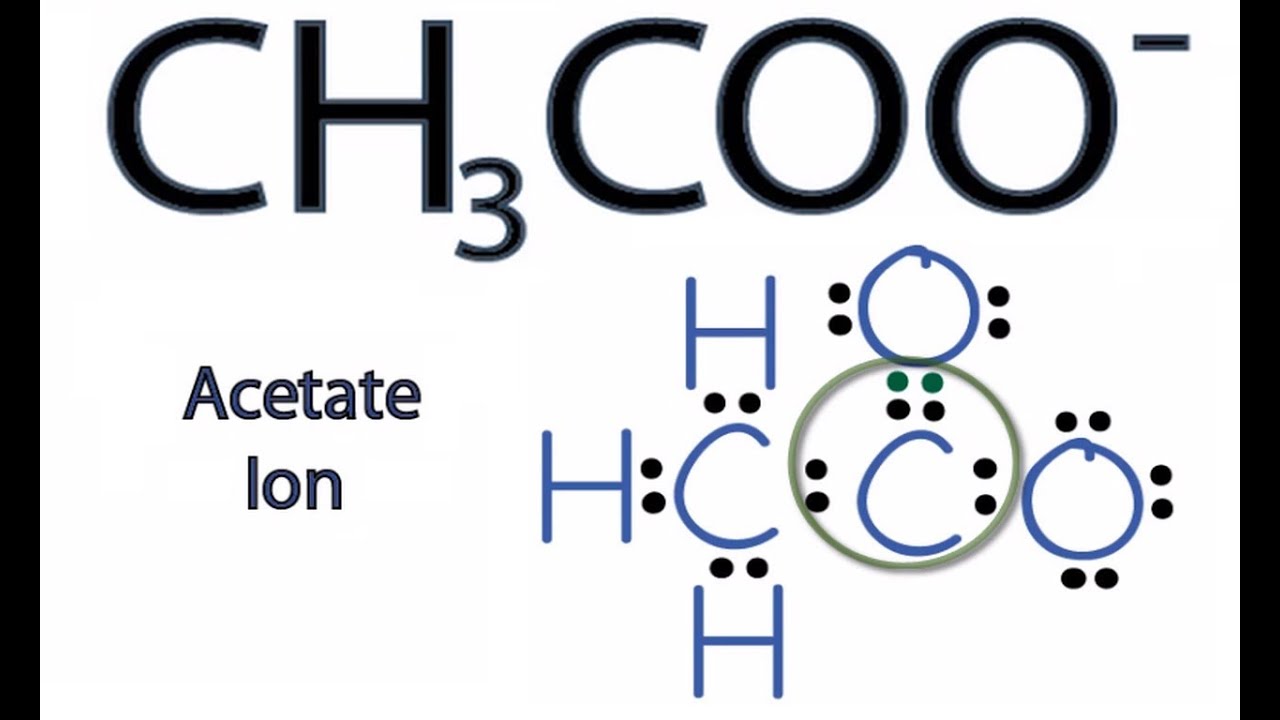
Resonance Structures For Ch3coo Acetate Ion Youtube

Draw The Lewis Structure Including Resonance Structures For The Acetate Ion Ch3coo For Each Brainly Com

Resonance Structure Of Ch3coo Brainly In

Solved Draw The Lewis Structure Including Resonance Chegg Com

Resonance Structures For Ch3coo Acetate Ion Youtube
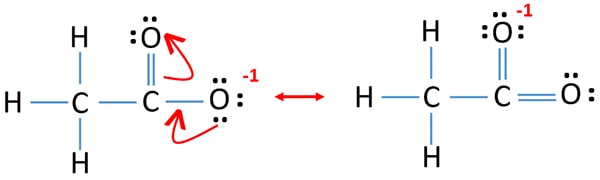
Acetate Ch3coo Ion Lewis Structure Resonance Structures
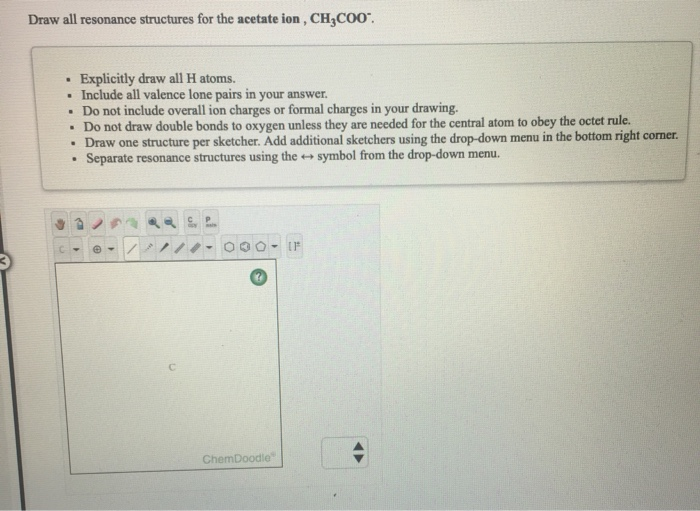
Solved Draw All Resonance Structures For The Acetate Ion Chegg Com
0 comments
Post a Comment